6.1 Introduction

It’s a hot August evening at a park in the middle of North Hudson, Wisconsin, a village of just under 4000 people on the St. Croix river in the western edge of the state. A line of people are seated at tables set up inside a canvas tent. In front of a cheering crowd of friends, family, and neighbours, these brave souls are about to do battle… with a fruit plate.
Unfortunately for the contestants, the fruit in question is the habanero, one of the hotter varieties of chili pepper commonly found in markets in North America. In this particular event, teams of five people will race to be the first to eat a full pound of peppers. As the eating begins, all seems well at first. Within 30 seconds, though, what begins to happen is completely predictable and understandable to anyone who has ever mistakenly poured a little too much hot sauce on the dinner plate. Faces turn red, sweat and tears begin to flow, and a copious amount of cold water is gulped down.
Although technically the contestants are competing against each other, the real opponent in this contest—the cause of all the pain and suffering—is the chemical compound “capsaicin,” the source of the heat in hot chili peppers.
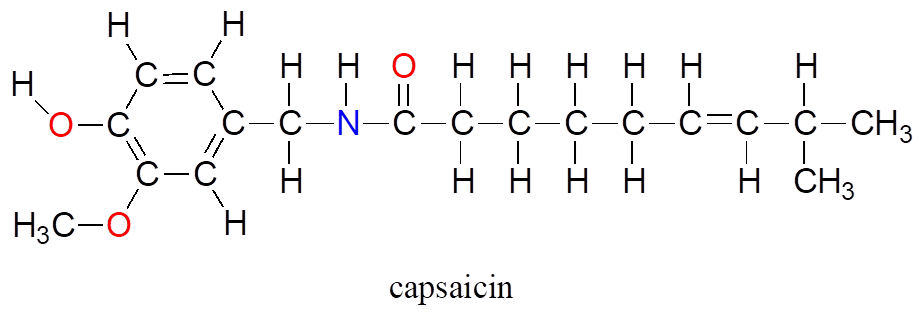
Composed of the four elements carbon, hydrogen, oxygen, and nitrogen, capsaicin is produced by the pepper plant for the purpose of warding off hungry mammals. The molecule binds to and activates a mammalian receptor protein called TrpV1, which in normal circumstances has the job of detecting high temperatures and sending a signal to the brain—”it’s hot, stay away!” This strategy works quite well on all mammalian species except one: we humans (some of us, at least) appear to be alone in our tendency to actually seek out the burn of the hot pepper in our food.
Interestingly, birds also have a heat receptor protein which is very similar to the TrpV1 receptor in mammals, but birds are not at all sensitive to capsaicin. There is an evolutionary logic to this: it is to the pepper’s advantage to be eaten by a bird rather than a mammal, because a bird can spread the pepper seeds over a much wider area. The region of the receptor which is responsible for capsaicin sensitivity appears to be quite specific—in 2002, scientists were able to insert a small segment of the (capsaicin-sensitive) rat TrpV1 receptor gene into the non-sensitive chicken version of the gene, and the resulting chimeric (mixed species) receptor was sensitive to capsaicin (Jordt & Julius, 2002, p. 421).
Back at the North Hudson Pepperfest, those with a little more common sense are foregoing the painful effects of capsaicin overload and are instead indulging in more pleasant chemical phenomena. A little girl enjoying an ice cream cone is responding in part to the chemical action of another organic compound called vanillin.
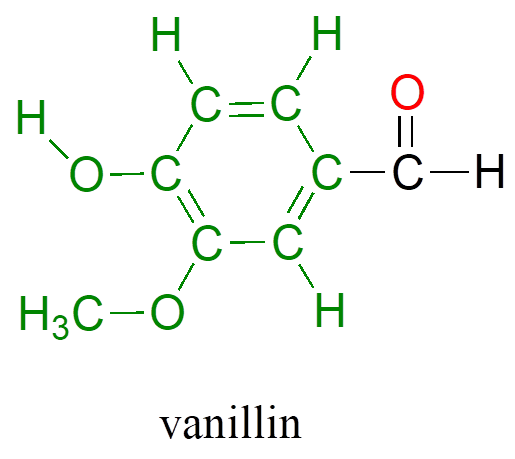
What is it about capsaicin and vanillin that causes these two compounds to have such dramatically different effects on our sensory perceptions? Both are produced by plants, and both are composed of the elements carbon, hydrogen, oxygen, and (in the case of capsaicin) nitrogen. Since the birth of chemistry as a science, chemists have been fascinated—and for much of that history, mystified—by the myriad properties of compounds that come from living things. The term “organic,” from the Greek organikos, was applied to these compounds, and it was thought that they contained some kind of “vital force” which set them apart from “inorganic” compounds such as minerals, salts, and metals, and which allowed them to operate by a completely different set of chemical principles. How else but through the action of a “vital force” could such a small subgroup of the elements combine to form compounds with so many different properties?
Today, as you are probably already aware, the term “organic”—when applied to chemistry—refers not just to molecules from living things, but to all compounds containing the element carbon, regardless of origin. Beginning early in the 19th century, as chemists learned through careful experimentation about the composition and properties of “organic” compounds such as fatty acids, acetic acid, and urea, and even figured out how to synthesize some of them starting with exclusively “inorganic” components, they began to realize that the “vital force” concept was not valid, and that the properties of both organic and inorganic molecules could in fact be understood using the same fundamental chemical principles.
They also began to more fully appreciate the unique features of the element carbon which makes it so central to the chemistry of living things, to the extent that it warrants its own subfield of chemistry. Carbon forms four stable bonds, either to other carbon atoms or to hydrogen, oxygen, nitrogen, sulfur, phosphorus, or a halogen. The characteristic bonding modes of carbon allow it to serve as a skeleton, or framework, for building large, complex molecules that incorporate chains, branches, and ring structures.
Although “organic chemistry” no longer means exclusively the study of compounds from living things, it is nonetheless the desire to understand and influence the chemistry of life that drives much of the work of organic chemists, whether the goal is to learn something fundamentally new about the reactivity of a carbon-oxygen bond, to discover a new laboratory method that could be used to synthesize a life-saving drug, or to better understand the intricate chemical dance that goes on in the active site of an enzyme or receptor protein. Although humans have been eating hot peppers and vanilla-flavoured foods for centuries, we are just now, in the past few decades, beginning to understand how and why one causes searing pain, and the other pure gustatory pleasure. We understand that the precise geometric arrangement of the four elements in capsaicin allows it to fit inside the binding pocket of the TrpV1 heat receptor—but, as of today, we do not yet have a detailed three-dimensional picture of the TrpVI protein bound to capsaicin. We also know that the different arrangement of carbon, hydrogen, and oxygen atoms in vanillin allows it to bind to specific olfactory receptors, but again, there is much yet to be discovered about exactly how this happens.
In this topic, you will be introduced to some of the most fundamental principles of organic chemistry. With the concepts we learn about, we can begin to understand how carbon and a very small number of other elements in the periodic table can combine in predictable ways to produce a virtually limitless chemical repertoire.
As you read through, you will recognize that the section contains a lot of review of topics you have probably learned already in an introductory chemistry course, but there will likely also be a few concepts that are new to you, as well as some topics which are already familiar to you but covered at a greater depth and with more of an emphasis on biologically relevant organic compounds.
We will begin with a reminder of how chemists depict bonding in organic molecules with the “Lewis structure” drawing convention, focusing on the concept of “formal charge.” We will review the common bonding patterns of the six elements necessary for all forms of life on Earth—carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus—plus the halogens (fluorine, chlorine, bromine, and iodine). We’ll then continue on with some of the basic skills involved in drawing and talking about organic molecules: understanding the “line structure” drawing convention and other useful ways to abbreviate and simplify structural drawings, learning about functional groups and isomers, and looking at how to systematically name simple organic molecules.
Before you continue any further in your reading, you should do some review of your own, because it will be assumed that you already understand some basic chemistry concepts. It would be a very good idea to revisit an introductory chemistry textbook or watch the excellent video tutorials at Khan Academy (see links below) to remind yourself about the following topics:
Key Topics to Review from Introductory Chemistry
From Khan Academy:
Additional Activities
Optional activity: For a comprehensive chart which outlines all of the major functional groups found in organic compounds, please see Compound Interest’s “Organic Functional Groups Chart – Expanded Edition.”
Watch the videos below which outline how to draw as well as identify functional groups in organic molecules.
Video: Organic Chemistry Functional Groups – How to Understand and Memorize Functional Groups (21 min. 33 sec.)
(Leah4sci, 2015)
Video: Identifying functional groups | Organic chemistry | Khan Academy (9 min. 17 sec.)
(Khan Academy Organic Chemistry, 2015)
Optional activity: For some interactive activities you can do to practise identifying functional groups online, please visit the following two sites: University of Sydney’s “Functional Groups Memory Game” and University of Illinois at Urbana-Champaign’s “Practice Functional Group Identification.”
Mini project: Identify five (5) biologically important molecules (may be molecules found in the body, or pharmaceutical compounds). Some examples you can use are acetyl coenzyme A, adenosine triphosphate (ATP), nicotinamide adenine dinucleotide (NAD), dopamine, acetylsalicylic acid, etc. On a poster, draw out your five selected molecules and circle and name all of the various functional groups you can find in each.
Atomic Structure, Electron Configuration, and Lewis Structure Review Exercises
Exercise 1:
How many neutrons do the following isotopes have?
- 31P, the most common isotope of phosphorus
- 32P, a radioactive isotope of phosphorus used often in the study of DNA and RNA
- 37Cl, one of the two common isotopes of chlorine
- Tritium (3H), a radioactive isotope of hydrogen, used often by biochemists as a “tracer” atom
- 14C, a radioactive isotope of carbon, also used as a tracer in biochemistry
Exercise 2:
The electron configuration of a carbon atom is 1s22s22p2, and that of a sodium cation (Na+) is 1s22s22p6. Show the electron configuration for:
- A nitrogen atom
- An oxygen atom
- A fluorine atom
- A magnesium atom
- A magnesium cation (Mg2+)
- A potassium atom
- A potassium ion (K+)
- A chloride anion (Cl–)
- A sulfur atom
- A lithium cation (Li+)
- A calcium cation (Ca2+)
Exercise 3:
Draw Lewis structures for the following species (use lines to denote bonds, and dots for lone-pair electrons). All atoms should have a complete valence shell of electrons. For now, do not worry about showing accurate bond angles.
- Ammonia, NH3
- Ammonium ion, NH4+
- Amide ion, NH2–
- Formaldehyde, HCOH
- Acetate ion, CH3COO–
- Methylamine, CH3NH2
- Ethanol, CH3CH2OH
- Diethylether, CH3CH2OCH2CH3
- Cyclohexanol (molecular formula C6H12O, with six carbons bonded in a ring and an OH group)
- Propene, CH2CHCH3
- Pyruvic acid, CH3COCO2H
Media Attribution
- Figure 1: Soderberg, T. (2019, July). Figure 1 [Digital image]. In Organic chemistry with a biological emphasis volume I. (Chapter 1). UMM Digital Well. https://digitalcommons.morris.umn.edu/chem_facpubs/1/ CC BY 4.0 (Original source of image by jeffreyw [username]. Habaneros [Digital image]. Flickr. https://www.flickr.com/photos/jeffreyww/9596051534/. Used under a CC BY 2.0 license.)
References
Jordt, S.-E. , & Julius, D. (2002). Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell, 108, 421–430. https://doi.org/10.1016/S0092-8674(02)00637-2
Khan Academy Organic Chemistry. (2015, May 10). Identifying functional groups | Organic chemistry | Khan Academy [Video]. YouTube. https://youtu.be/esJ5MbAHswc
Leah4sci. (2015, September 27). Organic chemistry functional groups – How to understand and memorize functional groups [Video]. YouTube. https://youtu.be/okv2us6pVxo
UMM Digital Well. (2019, July). Organic chemistry with a biological emphasis volume I. University of Minnesota. https://digitalcommons.morris.umn.edu/chem_facpubs/1/ CC BY-NC-SA 4.0.

