7.9 Effects of Enzyme Microenvironment on Acidity and Basicity
Virtually all biochemical reactions take place inside the active site pocket of an enzyme, rather than free in aqueous solution. The microenvironment inside an enzyme’s active site can often be very different from the environment outside in the aqueous solvent. Consider, for example, the side chain carboxylate on an aspartate residue in an enzyme. The literature pKa of this carboxylic acid group is listed as 3.9, but this estimate assumes that the group is positioned on the surface of the protein, exposed to water. In the physiological buffer of pH ~ 7, a carboxylic acid group with pKa = 3.9 will be fully deprotonated and negatively charged. If, however, an aspartate side chain happens to be buried deep inside the interior of the protein’s active site, and is surrounded primarily by nonpolar side chains such as alanine, phenylalanine, tryptophan, etc., the situation is very different.
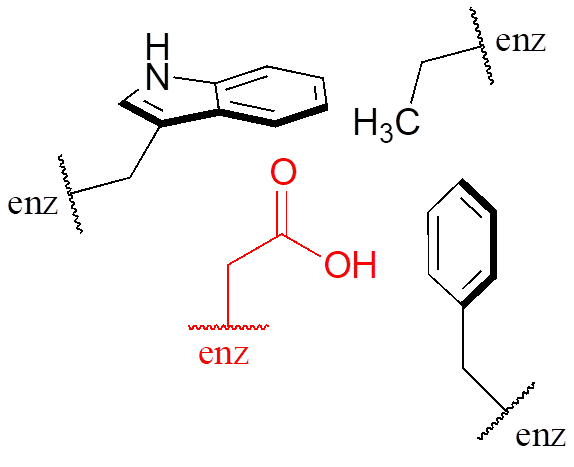
Cut off from the environment of the bulk solvent, the carboxylate group (red in the above figure) is now in a very nonpolar microenvironment, a situation in which the protonated, uncharged state is stabilized relative to the deprotonated, negatively charged state (this is simply another application of the “like dissolves like” principle—a charged group is highly destabilized by a nonpolar environment). The overall effect is that the pKa for this aspartate residue is actually higher than 3.9—it is less acidic, and more likely to be in its protonated form inside the protein.
A similar effect would be observed in a situation where the side chain carboxylate groups of two aspartate residues are located in close proximity to one another in an enzyme active site. Two negatively charged groups close to each other represents a very high-energy, repulsive situation, and this can be relieved if one of the two side chains is protonated.
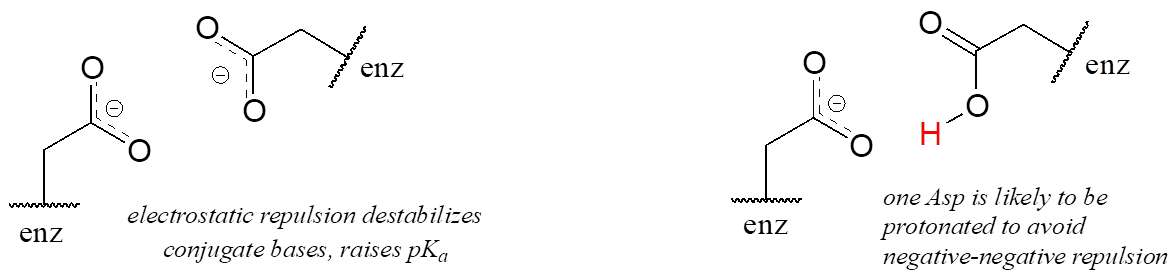
In this microenvironment, the proximity of one amino acid group directly affects the pKa of its neighbour.
Now consider a situation where a metal ion such as magnesium (Mg+2) or zinc (Zn+2) is bound in the interior of the enzyme, in close contact with an aspartate side chain. With a cation to interact with, the anionic, deprotonated state of the amino acid is stabilized, so the pKa of this Asp residue is likely to be substantially lower than 3.9.

The metal ion in this situation is considered to be acting as a Lewis acid, accepting electron density from the carboxylate group.
The pKa-lowering effect of a metal cation can be dramatic—it has been estimated that a water molecule coordinated to a Cu+2 or Zn+2 ion can have a pKa as low as 7 (compare this to the “normal” water pKa of 15.7!) (Groves et al., 1984).
Exercise 30:
A lysine residue located deep in the interior of a protein is surrounded by nonpolar residues. In what direction will this alter the “normal” pKa of the lysine side chain, and why?
Exercise 31:
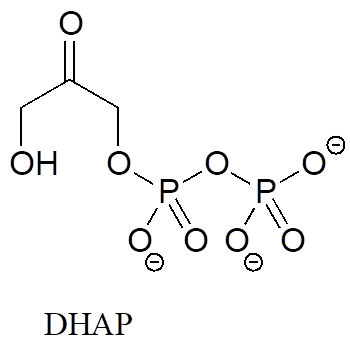
In many biochemical reactions which involve the formation of an enolate intermediate, the carbonyl oxygen of the substrate is coordinated to a divalent metal ion (usually zinc or magnesium) in the active site. Explain, with structural drawings, how this ion-dipole interaction affects the acidity of the α-protons of dihydroxyacetone phosphate (DHAP), an intermediate compound in the glycolysis pathway
Khan Academy: Video tutorials: organic acid-base chemistry
Summary of Key Concepts
Before you move on to the next section, you should:
- Know the Brønsted-Lowry definition of acidity and basicity: a Brønsted acid is a proton donor; a Brønsted base is a proton acceptor.
- Know the Lewis definition of acidity and basicity: a Lewis acid is an electron acceptor; a Lewis base is an electron donor.
- Understand that the Lewis definition is broader: all Brønsted acids are also Lewis acids, but not all Lewis acids are also Brønsted acids.
- Be able to draw a curved arrow mechanism for both Brønsted and Lewis acid-base reactions.
- Know the expressions for Ka and pKa.
- Commit to memory the approximate pKa values for the following functional groups:
- H3O+, protonated alcohol, protonated carbonyl (~ 0)
- Carboxylic acids (~ 5)
- Imines (~ 7)
- Protonated amines, phenols, thiols (~ 10)
- Water, alcohols (~ 15)
- α-carbon acids (~ 20)
- Be able to use pKa values to compare acidity: a lower pKa corresponds to a stronger acid.
- Know that:
- For a given pair of acids, the stronger acid will have the weaker conjugate base.
- For a given pair of basic compounds, the stronger base will have the weaker conjugate acid.
- Be able to identify the most acidic/basic groups on a polyfunctional molecule.
- Be able to calculate the equilibrium constant of an acid-base equation from the pKa values of the acids on either side of the equation.
- Be able to use the Henderson-Hasselbalch equation to determine the protonation state/charge of an organic compound in an aqueous buffer of a given pH.
- Understand the idea that the best way to compare the strength of two acids is to compare the stability of their conjugate bases: the more stable (weaker) the conjugate base, the stronger the acid.
- Be able to compare the acidity or basicity of compounds based on periodic trends:
- Acidity increases left to right on the table, so alcohols are more acidic than amines.
- Acidity increases top to bottom on the table, so a thiol is more acidic than an alcohol.
- Be able to compare the acidity or basicity of compounds based on protonation state: H3O+ is more acidic than H2O; NH4+ is more acidic than NH3.
- Understand how the inductive effect exerted by electronegative groups influences acidity.
- Understand how resonance delocalization of electron density influences acidity.
- Be able to explain/predict how orbital hybridization affects the relative acidity of terminal alkynes, alkenes, and alkanes.
- Be able to explain why phenols are more acidic than alcohols, and how electron-withdrawing or donating groups influence the acidity of phenols.
- Be able to identify the relative basicity of a nitrogen-containing group in a compound, based on whether it is an amine, amide, imine, aniline, or “pyrrole-like.”
- Be able to identify α-carbon(s) on a carbonyl compound, and explain why α-protons are weakly acidic. You should be able to draw the enolate conjugate base of a carbonyl compound.
- Be able to identify tautomeric relationships, specifically keto-enol and imine-enamine tautomers.
- Understand what a polyprotic acid is, what is meant by multiple pKa values, and why these values get progressively higher.
References
Groves, J. T., Rife, R., & Chambers, R., Jr. (1984). Geometrical and stereochemical factors in metal-promoted amide hydrolysis. Journal of the American Chemical Society, 106(3), 630–638. https://doi.org/10.1021/ja00315a030
UMM Digital Well. (2019, July). Organic chemistry with a biological emphasis volume I. University of Minnesota. https://digitalcommons.morris.umn.edu/chem_facpubs/1/ CC BY-NC-SA 4.0.

