8.2 Conformation of Organic Compounds
Conformations of Open-Chain Organic Molecules
Before we begin our exploration of stereochemistry and chirality, we first need to consider the subject of conformational isomerism, which has to do with rotation about single bonds.
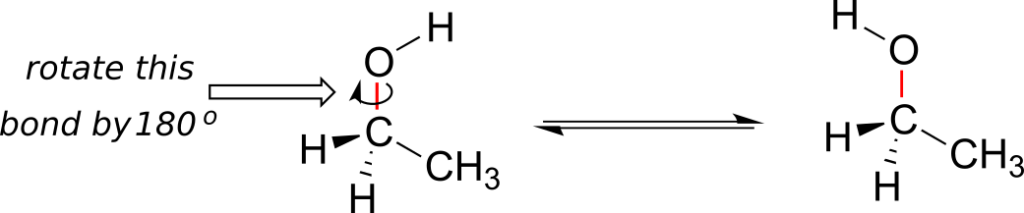
Single bonds in organic molecules are free to rotate, due to the “end-to-end” (σ) nature of their orbital overlap. Consider the carbon-oxygen bond in ethanol, for example: with a 180o rotation about this bond, the shape of the molecule would look quite different:
Or ethane: rotation about the carbon-carbon σ bond results in many different possible three-dimensional arrangements of the atoms.
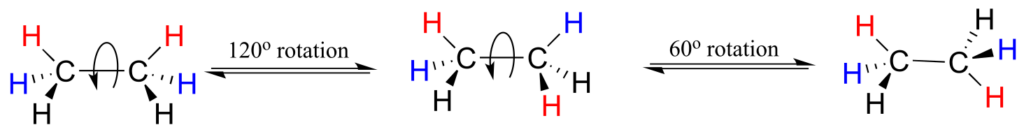
These different arrangements, resulting from σ bond rotation, are referred to in organic chemistry as conformations. Any one specific conformation is called a conformational isomer, or conformer.
In order to better visualize different conformations of a molecule, it is convenient to use a drawing convention called the Newman projection. In a Newman projection, we look lengthwise down a specific bond of interest—in this case, the carbon-carbon bond in ethane. We depict the “front” atom as a dot, and the “back” atom as a larger circle.
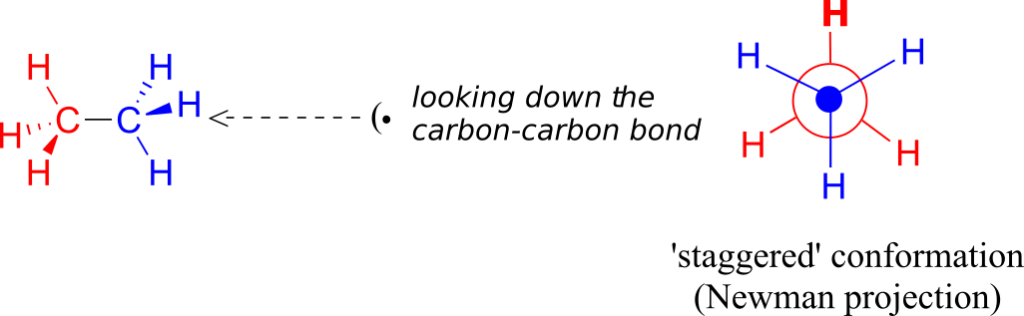
Interactive model: “Conformations of Ethane – Newman Projection” (ChemTube3D)
The six carbon-hydrogen bonds are shown as solid lines protruding from the two carbons. Note that we do not draw bonds as solid or dashed wedges in a Newman projection.
Looking down the C-C bond in this way, the angle formed between a C-H bond on the front carbon and a C-H bond on the back carbon is referred to as a dihedral angle. (The dihedral angle between the hour hand and the minute hand on a clock is 0o at noon, 90o at 3:00, and so forth).
The lowest energy conformation of ethane, shown in the figure above, is called the “staggered” conformation: all of the dihedral angles are 60o, and the distance between the front and back C-H bonds is maximized.
If we now rotate the front CH3 group 60° clockwise, the molecule is in the highest energy “eclipsed” conformation, where the dihedral angles are all 0o (we stagger the bonds slightly in our Newman projection drawing so that we can see them all).
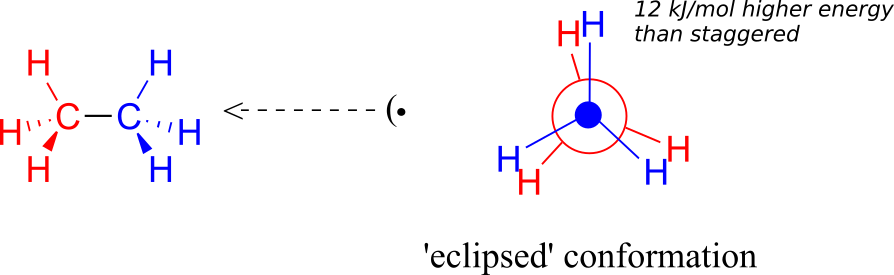
The energy of the eclipsed conformation, where the electrons in the front and back C-H bonds are closer together, is approximately 12 kJ/mol higher than that of the staggered conformation.
Another 60° rotation returns the molecule to a second staggered conformation. This process can be continued all around the 360° circle, with three possible eclipsed conformations and three staggered conformations, in addition to an infinite number of conformations in between these two extremes.
Video tutorial: Ethane Conformations and Newman Projections (fantzchem, 2013)
Now let’s consider butane, with its four-carbon chain. There are now three rotating carbon-carbon bonds to consider, but we will focus on the middle bond between C2 and C3. Below are two representations of butane in a conformation which puts the two CH3 groups (C1 and C4) in the eclipsed position, with the two C-C bonds at a 0o dihedral angle.
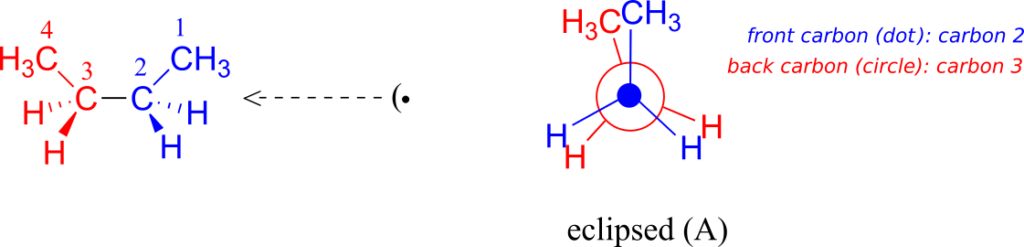
If we rotate the front (blue) carbon by 60° clockwise, the butane molecule is now in a staggered conformation.
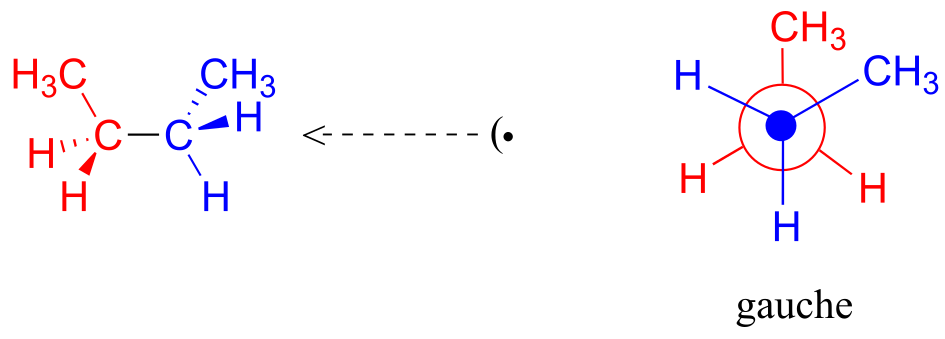
This is more specifically referred to as the gauche conformation of butane. Notice that although they are staggered, the two methyl groups are not as far apart as they could possibly be.
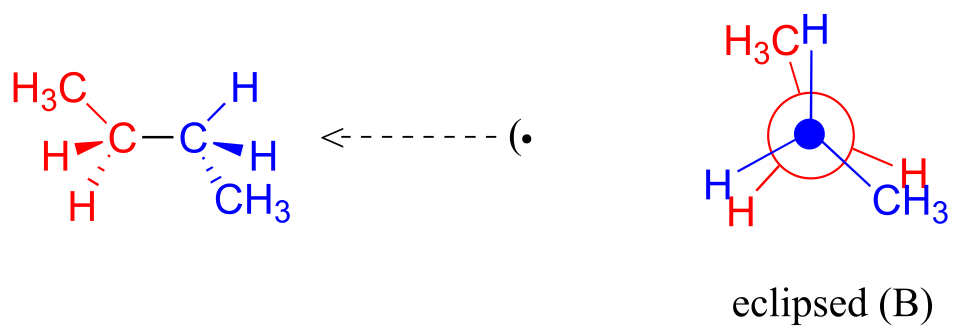
A further rotation of 60° gives us a second eclipsed conformation (B) in which both methyl groups are lined up with hydrogen atoms.
One more 60° rotation produces another staggered conformation called the anti conformation, where the two methyl groups are positioned opposite each other (a dihedral angle of 180°).
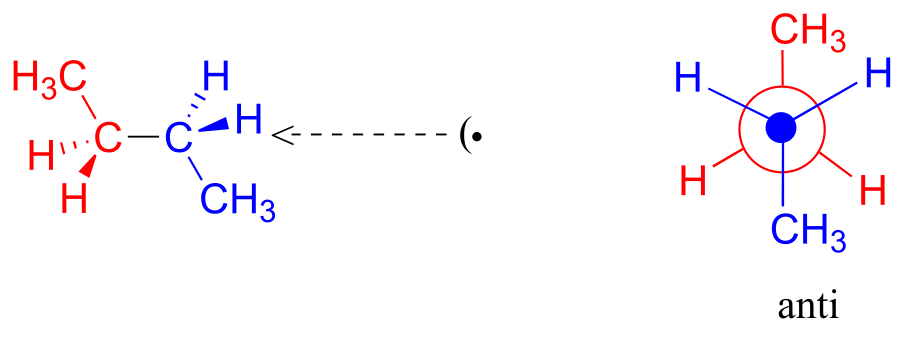
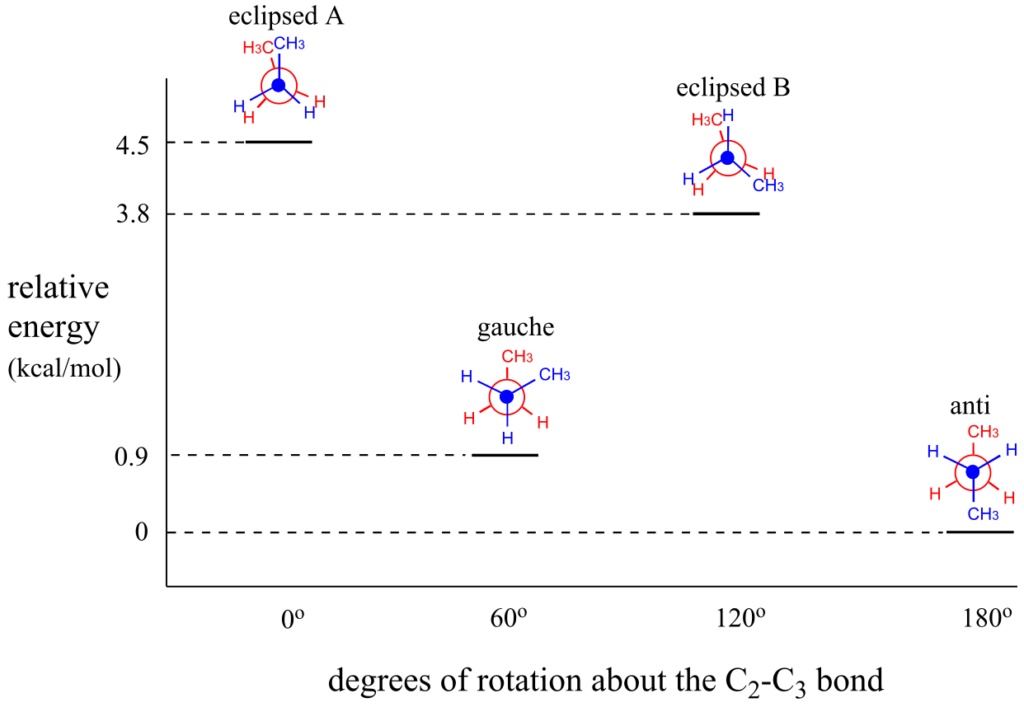
As with ethane, the staggered conformations of butane are energy “valleys,” and the eclipsed conformations are energy “peaks.” However, in the case of butane there are two different valleys, and two different peaks. The gauche conformation is a higher energy valley than the anti conformation due to steric strain, which is the repulsive interaction caused by the two bulky methyl groups being forced too close together. Clearly, steric strain is lower in the anti conformation. In the same way, steric strain causes the eclipsed A conformation—where the two methyl groups are as close together as they can possibly be—to be higher in energy than the two eclipsed B conformations.
The diagram below summarizes the relative energies for the various eclipsed, staggered, and gauche conformations of butane.
Interactive model: “Butane Newman Projections” (ChemTube3D)
Video tutorial: Butane Conformations (fantzchem)
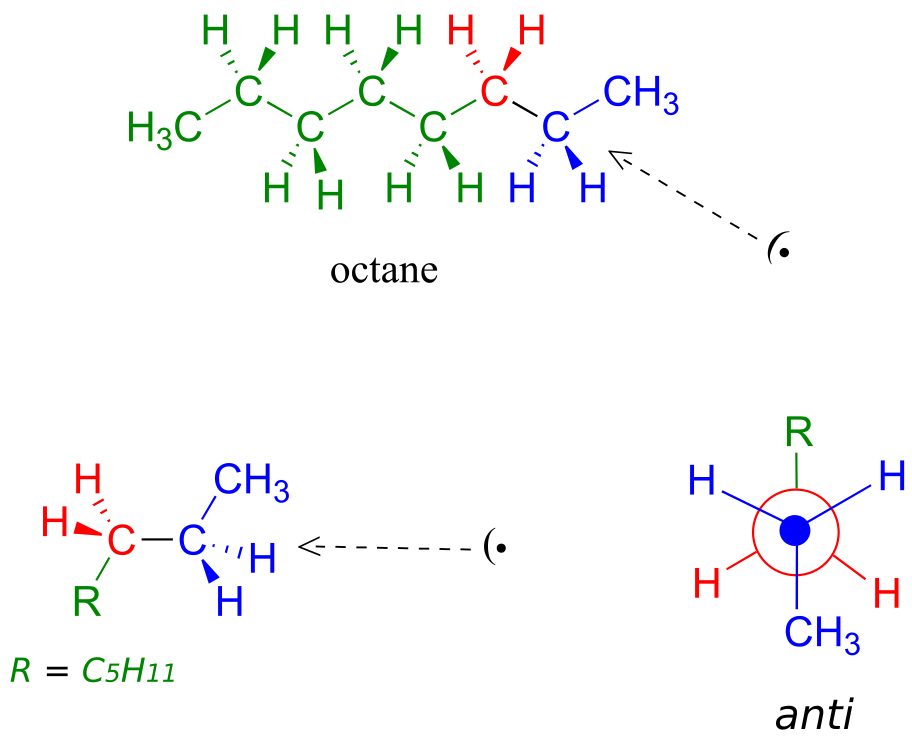
Because the anti conformation is lowest in energy (and also simply for ease of drawing), it is conventional to draw open-chain alkanes in a “zigzag” form, which implies anti conformation at all carbon-carbon bonds. The figure below shows, as an example, a Newman projection looking down the C2-C3 bond of octane.
Exercise 1:
Draw Newman projections of the lowest and highest energy conformations of propane.
Exercise 2:
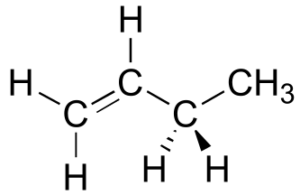
Draw a Newman projection, looking down the C2-C3 bond, of 1-butene in the conformation shown below (C2 should be your front carbon).
Conformations of Cyclic Organic Molecules
Browse through a biochemistry textbook and you will see any number of molecules with cyclic structures. Many of these cyclic structures are aromatic, and therefore planar. Many others, though, are composed of sp3-hybridized atoms, and it is these cyclic structures that are the topic of discussion in this section.
When discussing cyclic organic molecules, we will often use sugars as examples, because they are such important molecules in biological chemistry. It is important to recall that many sugars exist in aqueous solution as both open-chain and cyclic forms. You need not worry at this point about understanding how the cyclic form is named, or the reaction by which the cyclization occurs.
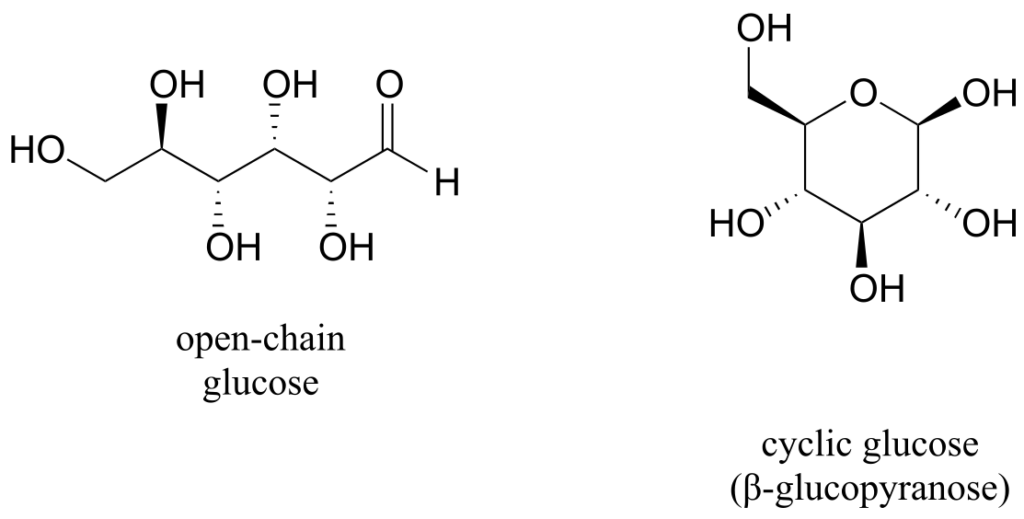
One thing that you should notice in the cyclic structure shown above is that atoms or groups bonded to tetrahedral ring carbons are either pointing up (out of the plane of the page) or down (into the plane of the page), as indicated by the use of dashed or solid wedge bonds. When two substituents on the same ring are both pointing toward the same side of the ring, they are said to be cis to each other. When they are pointed to opposite sides, they are said to be trans to each other.
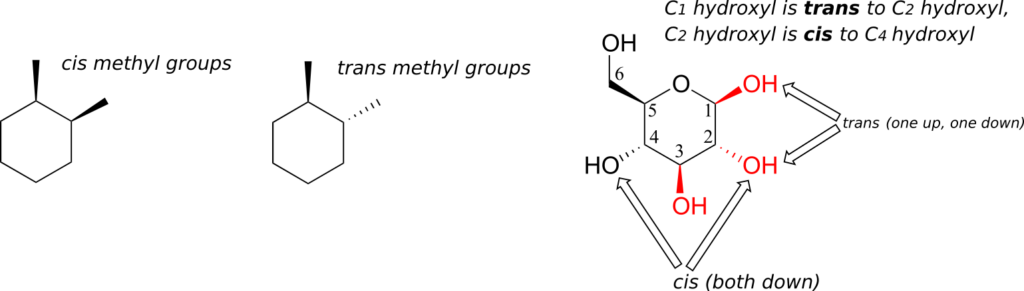
Ring structures in organic molecules are usually five-membered or six-membered. Three- and four-membered rings are occasionally found in nature, but are significantly higher in energy. The relative instability of these smaller ring structures can be explained by a concept called angle strain, in which the four bonds around the sp3-hybridized carbons are forced out of their preferred tetrahedral angles.
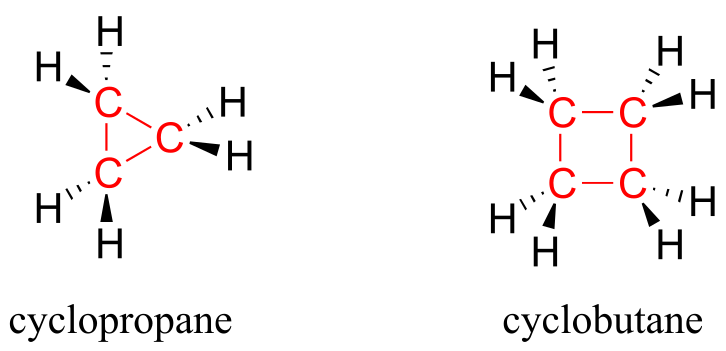
If one of the carbon-carbon bonds is broken, the ring will “spring” open, releasing energy as the bonds reassume their preferred tetrahedral geometry. The effectiveness of two antibiotic drugs, fosfomycin and penicillin, is due in large part to the high reactivity of the three- and four-membered rings in their structures.
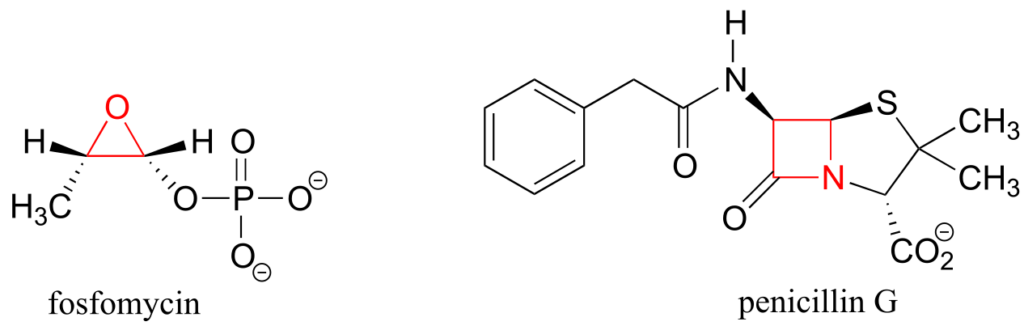
In six-membered cycloalkane structures, bonding angles are close to tetrahedral, and thus ring strain is not a factor—these rings are in fact very stable. However, the “flat” drawings we have been using up to now do not accurately show the actual three-dimensional shape of a five- or six-membered ring. If cyclohexane were indeed flat, the bond angles would have to be distorted from 109.5° to 120°. If you build a model, though, you will find that when you rotate the carbon-carbon bonds so as to put the ring into a shape that resembles a reclining beach chair, all of the carbon-carbon bonds are able to assume tetrahedral bonding angles.
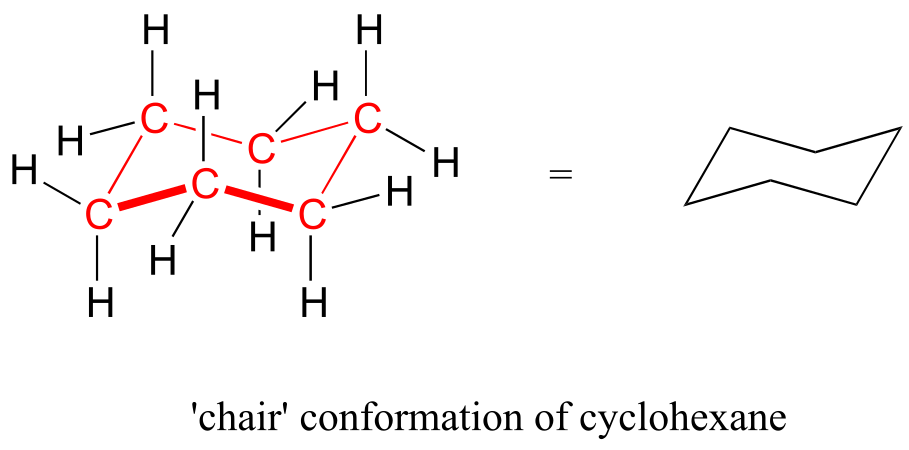
Interactive model: “Conformations of Cyclohexane – Chair and Boat” (ChemTube3D)
This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings.
An alternate conformation for a six-membered ring is called the “boat“:
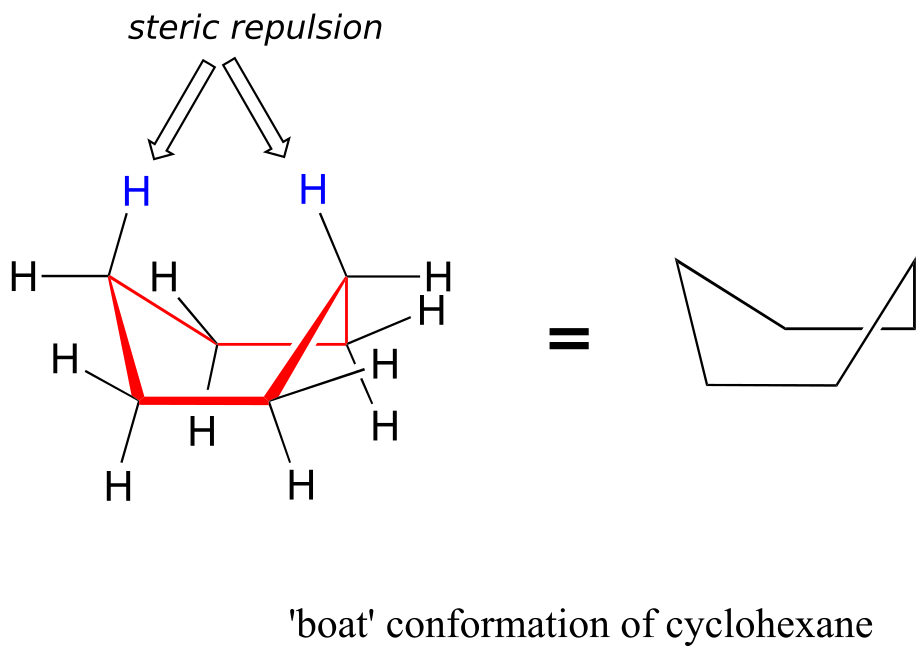
In the boat conformation, two of the substituents—those on the “bow” and the “stern” if you will—are brought close enough to each other to cause steric strain. An additional cause of the higher energy of the boat conformation is that adjacent hydrogen atoms on the “bottom of the boat” are forced into eclipsed positions. For these reasons, the boat conformation is a high-energy conformation of cyclohexane, about 30 kJ/mol less stable than the chair conformation.
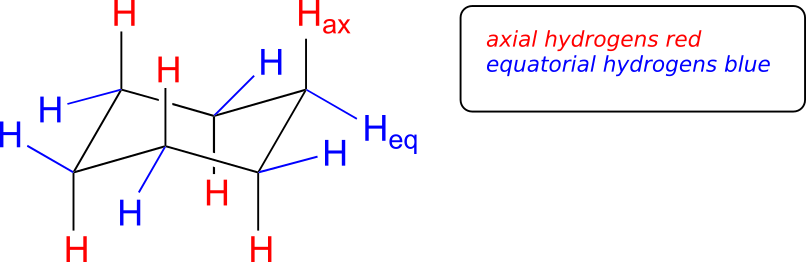
If you look carefully at your model of cyclohexane in the chair conformation, you will see that all 12 hydrogens are not equivalent in terms of their three-dimensional arrangement in space. Six hydrogens are axial—that is, they are pointing either straight up or straight down relative to the ring. The other six hydrogens are equatorial, meaning that they are pointing away from the perimeter of the ring, either slightly up or slightly down. (The equatorial vs. axial distinction is often hard to see at first—it would be a very good idea at this point to sit down with your instructor or tutor and work with a modelling kit).
This is not the only possible chair conformation for cyclohexane. On your model, rotate one of the “up” carbons down, and one of the “down” carbons up. You now have a new, alternate chair conformation—this process is called ring inversion.
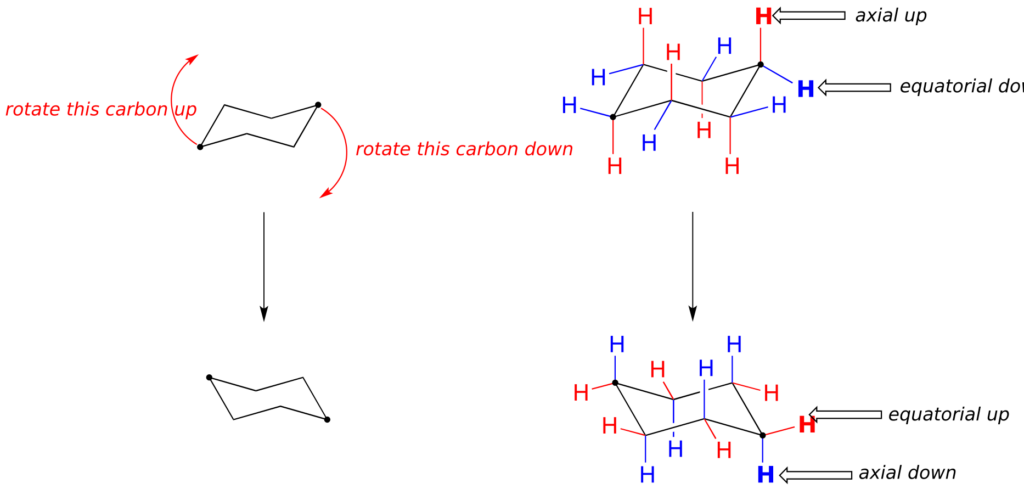
What you should recognize here is that, as a result of the ring inversion process, all of the axial and equatorial hydrogens have traded positions—axial hydrogens have become equatorial, and vice-versa. Notice, however, that the “down” hydrogens are still pointing down, and the “up” hydrogens are still pointing up regardless of whether they are axial or equatorial. At room temperature, cyclohexane is constantly inverting between two chair forms of equal energy—it is a rapid equilibrium situation. Thus, except at very low temperatures, we are not able to distinguish between axial and equatorial hydrogens, as they are constantly switching back and forth.
axial/equatorial vs. cis/trans
A very common error made by organic chemistry students as they begin to learn about chair conformations is to confuse the terms axial and equatorial with the terms cis and trans. These are completely different things! For example, when two substituents on a ring are cis in relation to one another, it means that they are pointed to the same side of the ring (both up or both down). Depending on their positions on the ring, they might both be axial, both be equatorial, or be one of each.
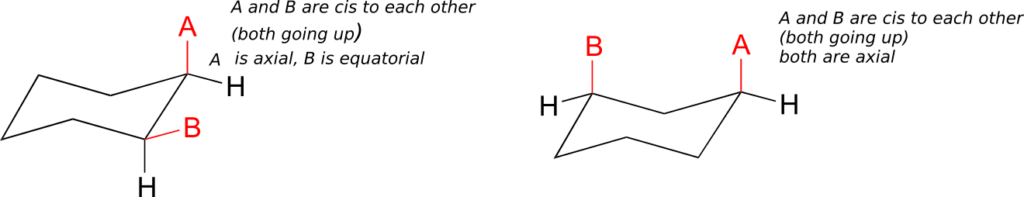
Do not make the mistake of calling two substituents trans to each other merely because one is equatorial and one is axial, or cis because the are both axial or both equatorial.
How to draw the cyclohexane chair conformation:
As an organic chemistry student, you will be expected to be able to draw an accurate representation of the chair conformations of six-membered cycloalkanes, which includes being able to draw axial and equatorial substituents with their correct orientations. Here, then, are some guidelines to follow:
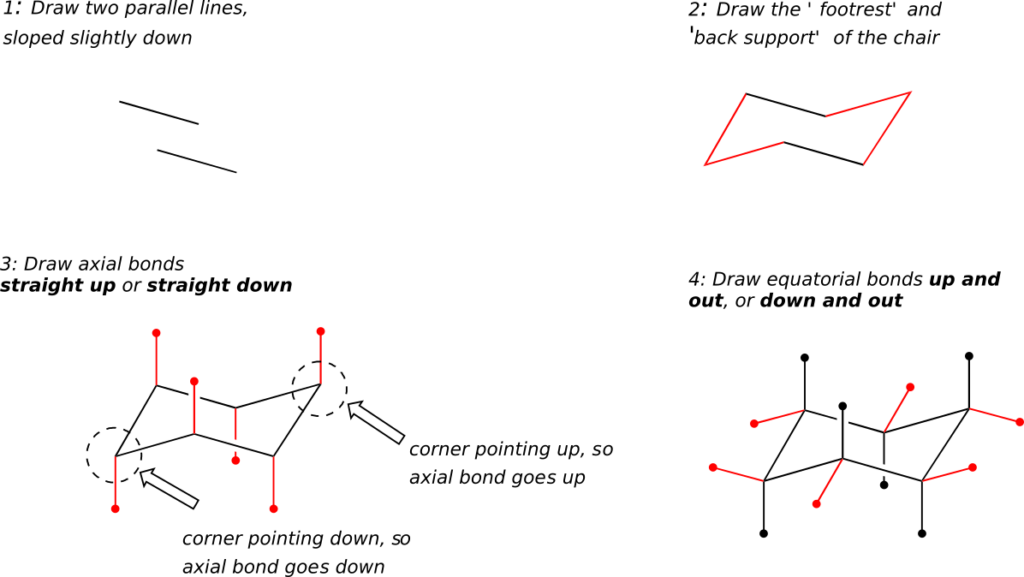
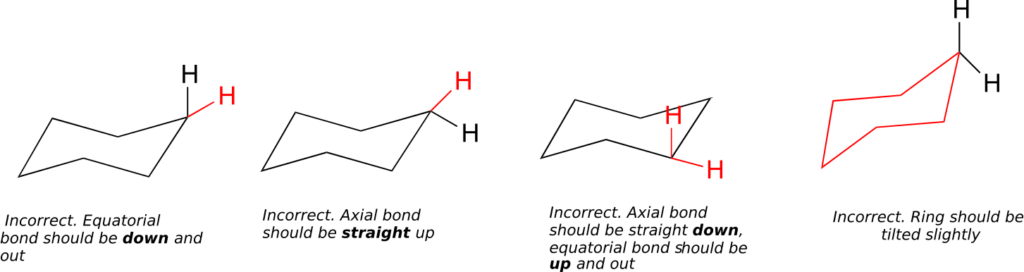
(How not to draw the chair):
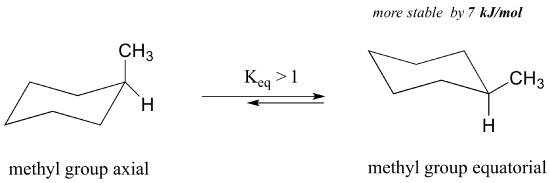
What happens to the relative energies of chair conformations when the ring has a large substituent, such as a methyl group? Now, the two chair conformations are quite different: in one, the methyl group is equatorial, and in the other it is axial.
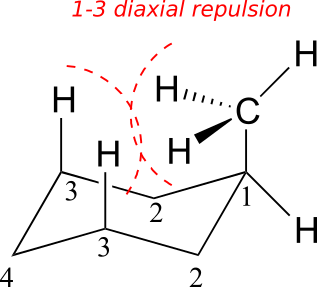
When the methyl group is in the axial position, it is brought close enough to the axial hydrogens on carbons two bonds away to cause destabilizing steric repulsion: this is referred to as 1,3-diaxial repulsion.
When in the equatorial position, the methyl group is pointing up and away from the rest of the ring, eliminating the unfavourable 1,3-diaxial interaction. As a consequence, the conformation in which the methyl group is in the equatorial position is more stable, by approximately 7 kJ/mol. At room temperature, methylcyclohexane exists as a rapid equilibrium between the two chair forms (and many other intermediate conformations), but the equilibrium constant (Keq) favours the conformation where the methyl group is equatorial.
Exercise 3:
Here’s some general chemistry review: what is the value of Keq at 25 °C for the axial to equatorial interconversion of methylcyclohexane as shown in the previous figure?
The importance of the steric strain factor increases with the increasing size of a substituent. For example, the difference in energy between the two chair conformations of tert-butyl cyclohexane (24 kJ/mol) is much larger than for methylcyclohexane (7 kJ/mol), because a tert-butyl group is larger than a methyl group and results in more energetically unfavourable 1,3-diaxial interactions.
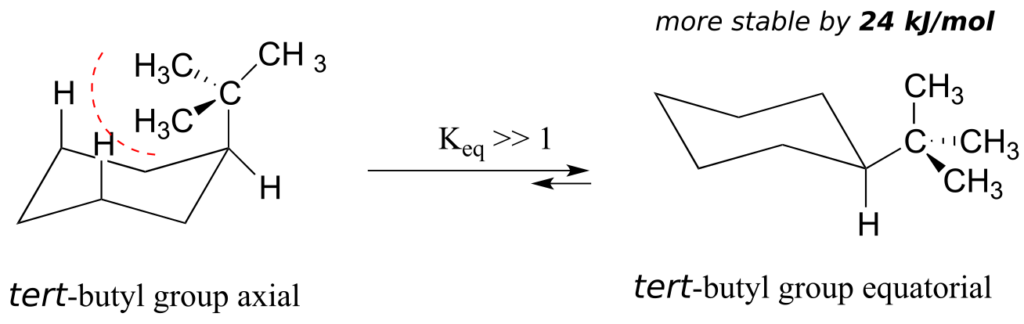
In the case of a disubstituted cyclohexane ring in which both substituents cannot be equatorial, the lower-energy conformation generally places the bulkier substituent in the equatorial position.
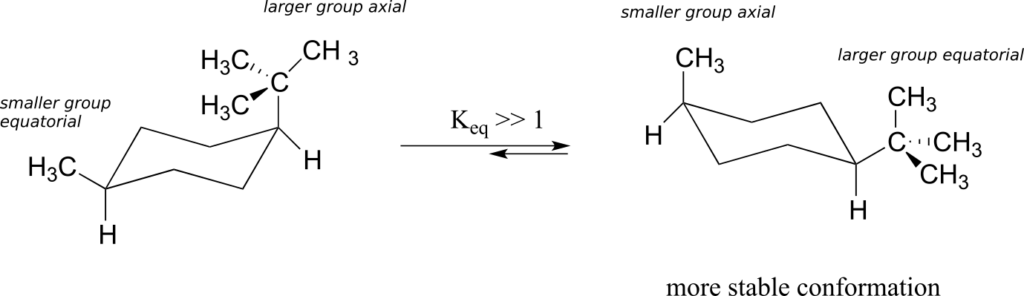
Exercise 4:
Draw the lower-energy chair conformations of a) trans-1,2-dimethylcyclohexane, and b) trans-1-isopropyl-3-methylcyclohexane. Draw all substituents on all carbons (including hydrogens), being sure that the axial or equatorial orientation is clear. Be sure to check your drawing with your instructor or tutor.
Exercise 5:
Predict which of the following disubstituted hexanes has a greater energy difference between its two chair conformations, and state your reasons for your choices.
- cis-1,3-dimethylcyclohexane or cis-1,4-dimethylcyclohexane
- cis-1,2-dimethylcyclohexane or trans-1,2-dimethylcyclohexane
- trans-1,2-dimethylcyclohexane or trans-1-isopropyl-2-methylcyclohexane
Exercise 6:
Can a “ring inversion” change a cis-disubstituted cyclohexane to trans? Explain.
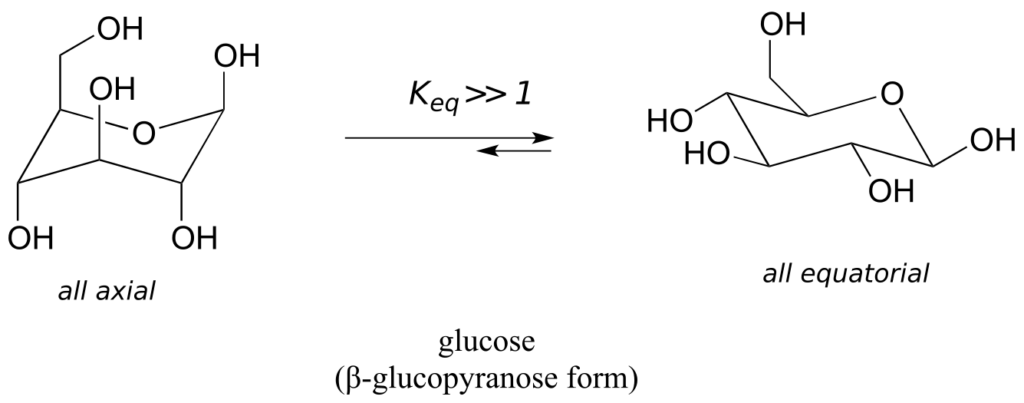
Recall that five- and six-carbon sugars such as glucose and fructose exist in solution in open chain and cyclic forms. Glucose, in its most abundant form in solution, is a six-membered ring adopting a chair conformation with all substituents equatorial.
The most abundant form of fructose in aqueous solution is also a six-membered ring.
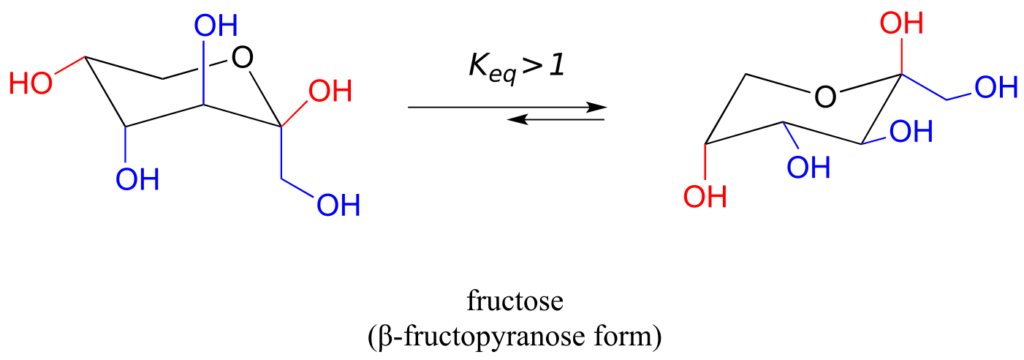
The lower-energy chair conformation is the one with three of the five substituents (including the bulky –CH2OH group) in the equatorial position.
Exercise 7:
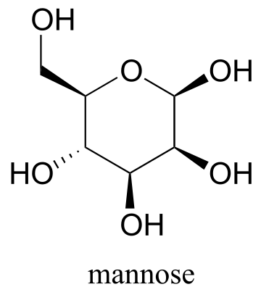
Draw the two chair conformations of the six-carbon sugar mannose, being sure to clearly show each non-hydrogen substituent as axial or equatorial. Predict which conformation is likely to be more stable, and explain why.
Video tutorial: Chair and Boat Shapes for Cyclohexane (Khan Academy)
The lowest-energy conformation of cyclopentane and other five-membered rings is known as the “envelope,” with four of the ring atoms in the same plane and one out of plane (notice that this shape resembles an envelope with the flap open). The out-of-plane carbon is said to be in the endo position (“endo” means “inside”).
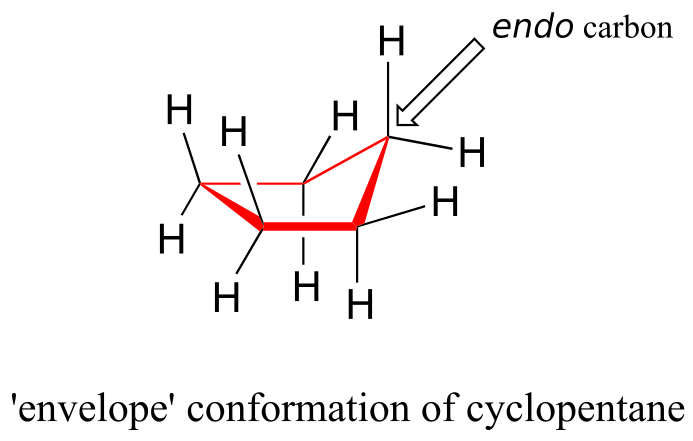
The “equatorial” vs. “axial” distinction discussed in the context of six-membered rings does not apply to five-membered rings.
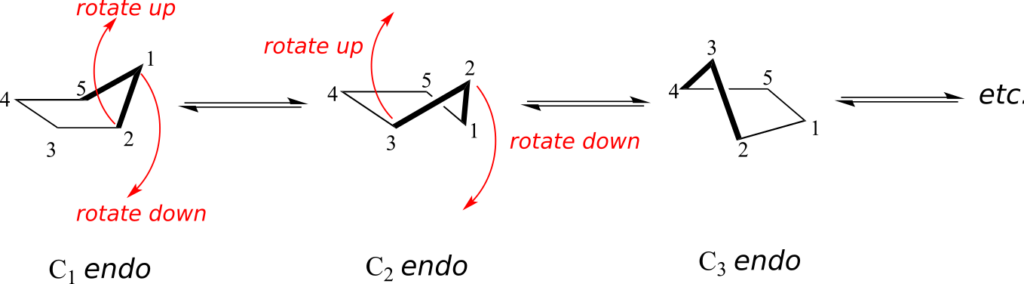
At room temperature, cyclopentane undergoes a rapid pseudorotation process in which each of the five carbons takes turns being in the endo position.
One of the most important five-membered rings in nature is a sugar called ribose—DNA and RNA are both constructed upon “backbones” derived from ribose. Pictured below is one thymidine (T) deoxy-nucleotide from a stretch of DNA:
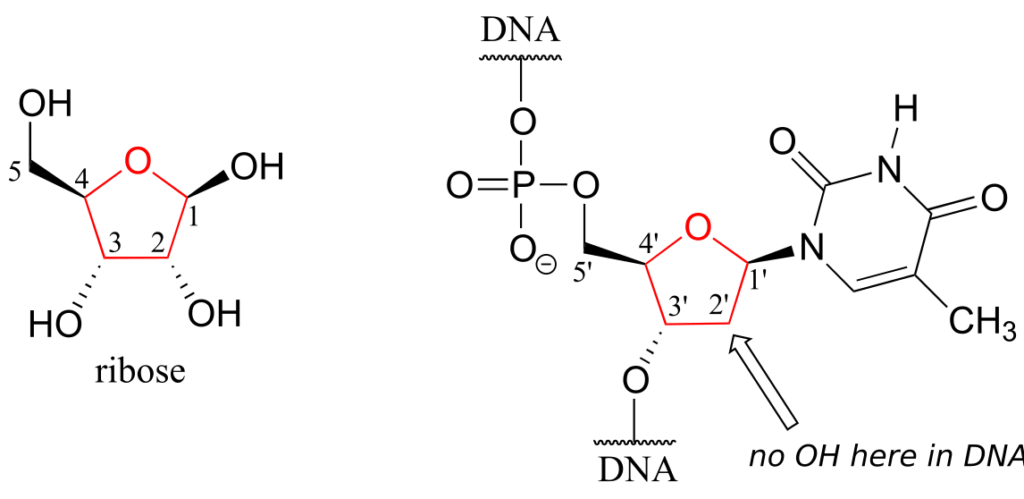
The lowest-energy conformations for ribose are envelope forms in which either the 3‘ or 2‘ carbons are endo. This has very important implications for oligonucleotide structure—in a DNA double helix, it is C2 that is in the endo position, while in RNA it is C3.

