7.8 Polyprotic Acids
Polyprotic acids are capable of donating more than one proton. The most important polyprotic acid group from a biological standpoint is triprotic phosphoric acid. Because phosphoric acid has three acidic protons, it also has three pKa values.

The pKa values for any polyprotic acid always get progressively higher, because it becomes increasingly difficult to stabilize the additional electron density that results from each successive proton donation. H3PO4 is a strong acid because the (single) negative charge on its conjugate base H2PO4– can be delocalized over two oxygen atoms.
H2PO4– is substantially less acidic, because proton donation now results in the formation of an additional negative charge: a –2 charge is inherently higher in energy than a –1 charge because of negative-negative electrostatic repulsion. The third deprotonation, resulting in formation of a third negative charge, has an even higher pKa.
Exercise 29:
In a buffer at physiological pH, what form(s) of phosphoric acid predominate(s)? What is the average net charge?
Free amino acids are polyprotic, with pKa values of approximately 2 for the carboxylic acid group and 9–10 for the ammonium group. Alanine, for example, has the acid constants pKa1 = 2.3 and pKa2 = 9.9.
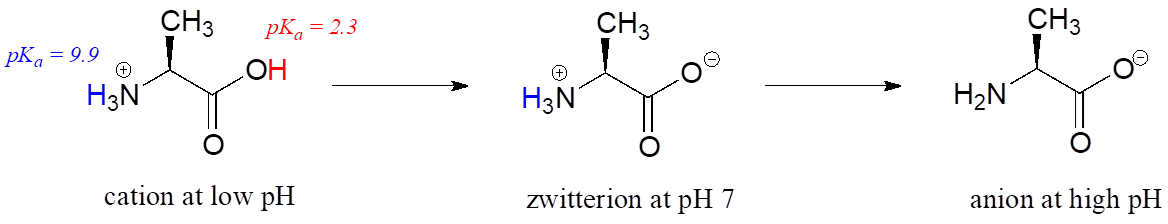
The Henderson-Hasselbalch equation tells us that alanine is almost fully protonated and positively charged when dissolved in a solution that is buffered to pH 0.5. At pH 7, alanine has lost one proton from the carboxylic acid group, and thus is a zwitterion (it has both a negative and a positive formal charge). At pH levels above 12, the ammonium group is fully deprotonated, and alanine has a negative overall charge.
Some amino acids (arginine, lysine, aspartate, glutamate, tyrosine, and histidine) are triprotic, with a third pKa value associated with an ionizable functional group on the side chain.
Many biological organic molecules have several potentially ionizable functional groups and thus can be considered polyprotic acids. Citric acid, found in abundance in oranges, lemons, and other citrus fruits, has three carboxylic acid groups and pKa values of 3.1, 4.8, and 6.4.
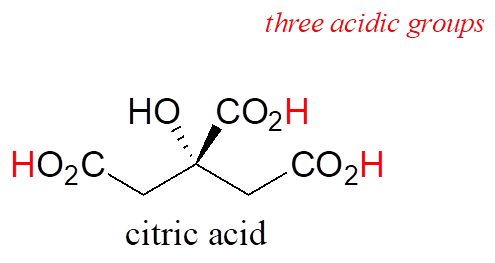
Reference
UMM Digital Well. (2019, July). Organic chemistry with a biological emphasis volume I. University of Minnesota. https://digitalcommons.morris.umn.edu/chem_facpubs/1/ CC BY-NC-SA 4.0.

